Trial AMPlify Designs a Multi Language Clinical Trial Site for Vivet
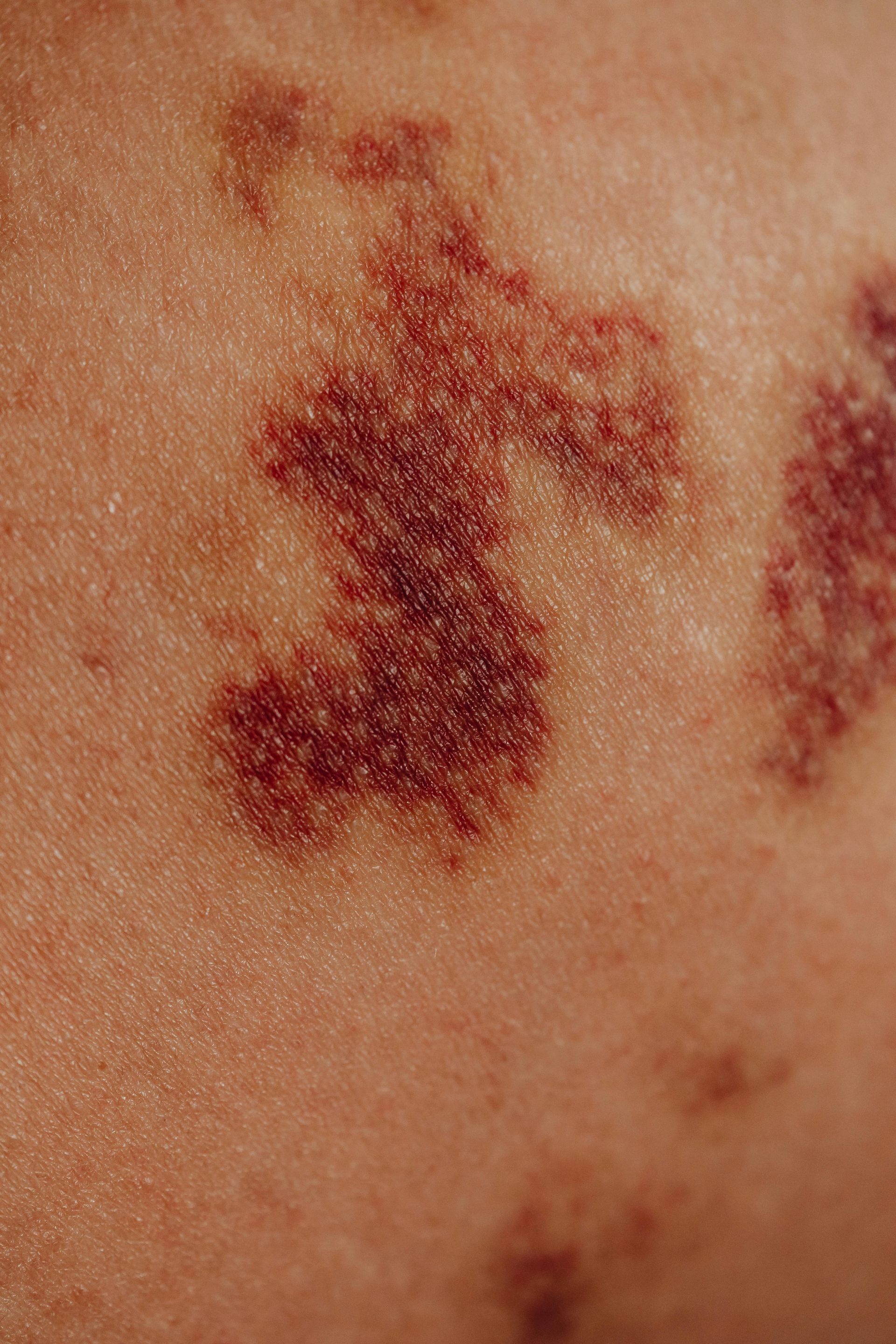
Trial AMPlify, a leading clinical trial digital advertising agency located in Boston, MA, designed and developed a multi-language website to promote a new Wilson's Disease gene therapy study across the US and Europe.
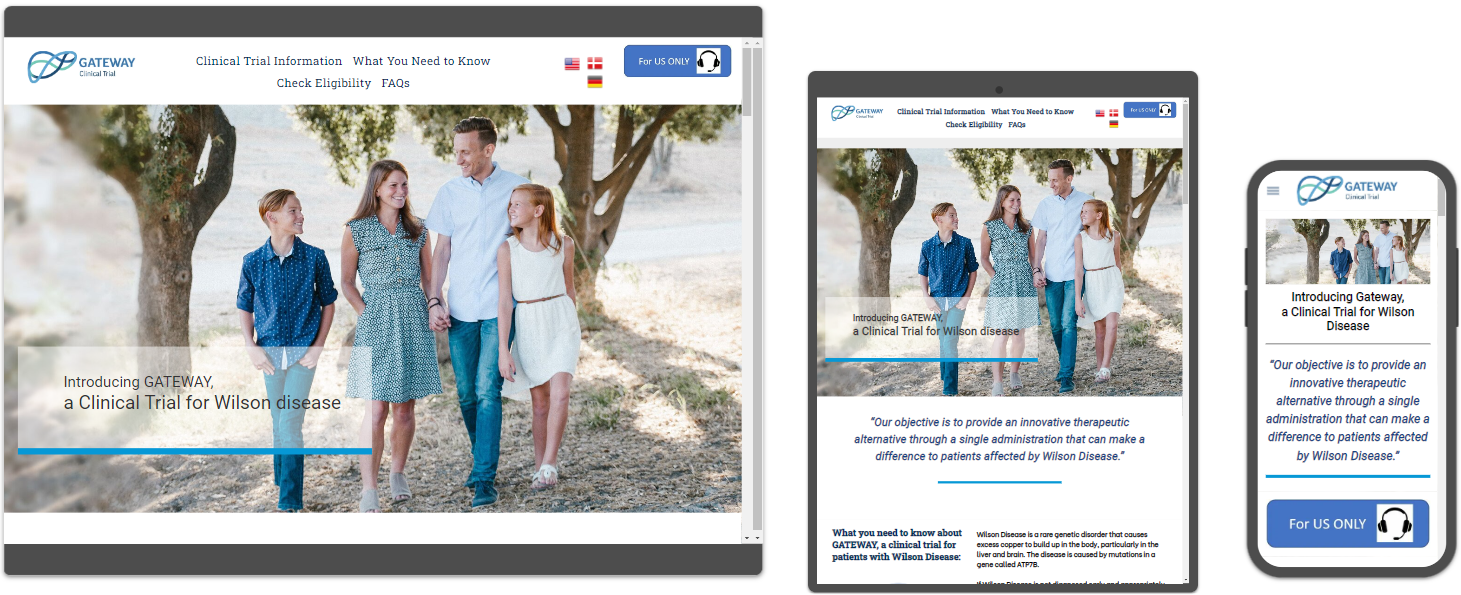
Trial AMPlify, a division of Boston Web Partners, launches a new multi-language clinical trial website for Vivet Therapeutics to study a new gene therapy to address Wilson's Disease. The disease is caused by mutations in a gene called ATP7B. VTX-801.
The website design was developed on an easy to edit platform without the need for constant plugin maintenance or theme updates. Site is hosted on Amazon (AWS) servers to ensure fast page loading. 90% of the Top 10 global biopharma companies use AWS.
Unlike other web design agencies, Trial AMPlify is able to eliminate the concern over WordPress malware attacks, and GDPR plugin compliance issues.
SEO best practices were implemented to help the site to rank higher in Google searches over time. While SEO is taking hold, a Google Ads campaign was launched to help drive web visitor traffic to the site immediately to increase subject enrollment across the US and Europe.
>> Click here to view the site - www.gatewaytrialwilsondisease.com