Clinical Trial Marketing Best Practices for 2025
Patient Recruitment Clinical Trial Marketing in 2025. What Works, What Doesn’t… and the Two Biggest Mistakes Sponsors Keep Making.

Look into this patient's eyes. He has just been diagnosed with a debilitating disease that will soon change his life. He has questions and wants answers - FAST. Sponsors and CROs will hope to connect with him on Facebook to make him aware of their clinical trial. Trouble is, when he gets home, he will scour Google, not Facebook, for the next five hours to find the answers he needs. He doesn't even have a Facebook account.
After seeing claims for acupuncture, herbs and unsuccessfully trying to navigate the archaic interface of ClincalTrials.gov, he starts to lose hope. What he and his physician are both unaware of is that there is a clinical trial recruiting right now for his condition with a site ten miles away. Sadly, this is not an unfamiliar story due to the lack of effective clinical trial marketing. Trial AMPlify, a patient recruitment company located in Boston, is on a mission to change how sponsors create awareness for their trials.
As of January 2024, the lack of marketing funds allocated to promote clinical trials is breathtakingly low or non-existent. As a result, 85% of patients are unaware that participation in a clinical trial is an option at the time of diagnosis. Based on a Deloitte analysis, the cost to bring a drug to market is now over two billion dollars. Despite this considerable investment, sponsors and CROs are skipping multiple steps in the marketing ecosystem that would make patients, HCPs, advocacy groups, and physicians aware of their trials.
Mistake # 1
Sponsors are still under the illusion that all of their potential patients are flocking to Facebook and logging in regularly to draw their knowledge from online advocacy groups. Here are some facts to consider: 1) Newly diagnosed people are on Google. These patients have the most hope. 2) Advice, opinions, and misconceptions on a Facebook group can muddy the conversation. Rally cries around new treatments can be debated and challenged rather than revered. 3) Dissenters in the group can discourage others from joining a trial. This is why other marketing channels should also be leveraged for better coverage and exposure.
Effective patient recruitment marketing techniques can vary depending on the specific goals, target audience, and resources of a clinical trial or healthcare organization. However, here are some commonly used and effective techniques:
Digital Marketing:
- Google Ads (Pay per Click): Use pay-per-click (PPC) advertising to target specific keywords related to your clinical trial, ensuring that your trial appears in relevant search results. Google advertising can be the best channel of them all since this is the first place new patients go to discover more about their condition, options and -- stubble upon a clinical trial tailored specifically for their condition.
- Search Engine Optimization (SEO):
Promote Google page # 1 rankings for all the keywords around your study. By optimizing your website and content, you can rank higher in organic search results for relevant keywords. Creating high-quality blog posts, articles, and videos that provide valuable information about the condition being studied and the benefits of participating in the trial can help with this.
- Display Advertising:
Excellent for laser-targeting physicians (Trial
AMPlify
has access to over 400K physicians we can build awareness with worldwide). Targeting the specific type of doctors you're looking to influence can increase buy-in and promote organic physician-to-patient communication and enrollment.
- And Yes, Social Media Advertising:
Utilize platforms like Facebook, Instagram, and X (formerly known as Twitter) to target specific advocacy groups, demographics and interests that match your trial's criteria.
- Fear Not:
It's important to note that all these marketing communications are widely used and can be transparent, accurate, and respectful of patient privacy. Tailoring the approach with an experienced patient recruitment agency based on the specific clinical trial, target demographic, and the nature of the medical condition being studied is also key to successful patient recruitment.
All of these clinical trial marketing strategies coupled with a patient-centric website is by far the best marketing approach. Incorporating patient stories on the website is also
beneficial. Authentic and relatable stories can inspire others to join the study. The most effective patient recruitment marketing techniques may require a combination of these strategies, tailored to the specific needs and characteristics of your clinical trial and target population. Regularly evaluate the performance of your marketing efforts and be prepared to adjust your tactics as needed to achieve your subject recruitment goals.
Mistake # 2
The second biggest mistake sponsors are making is using their ClinicalTrials.gov listing as the sole source of information for their protocol. Do we truly expect that potential subjects will find, understand and become excited enough to reach out, and join the trial. Creating a dedicated clinical trial website is 74% more effective at conveying the study. What's more, the new study site can be the destination patients visit for all the marketing channels listed above.
More needs to be done to move the needle in terms of building awareness, credibility and trust from a patient marketing perspective. Relying on Facebook and your CT.gov listing will ultimately leave the study and its breakthrough science adrift resulting in a failure to meet its patient enrollment targets. The mindset around clinical trial marketing needs to change if studies are to thrive in 2024 and beyond.
"We know from our research that it only takes one friend or family member to scare a patient away from enrolling in a clinical trial."
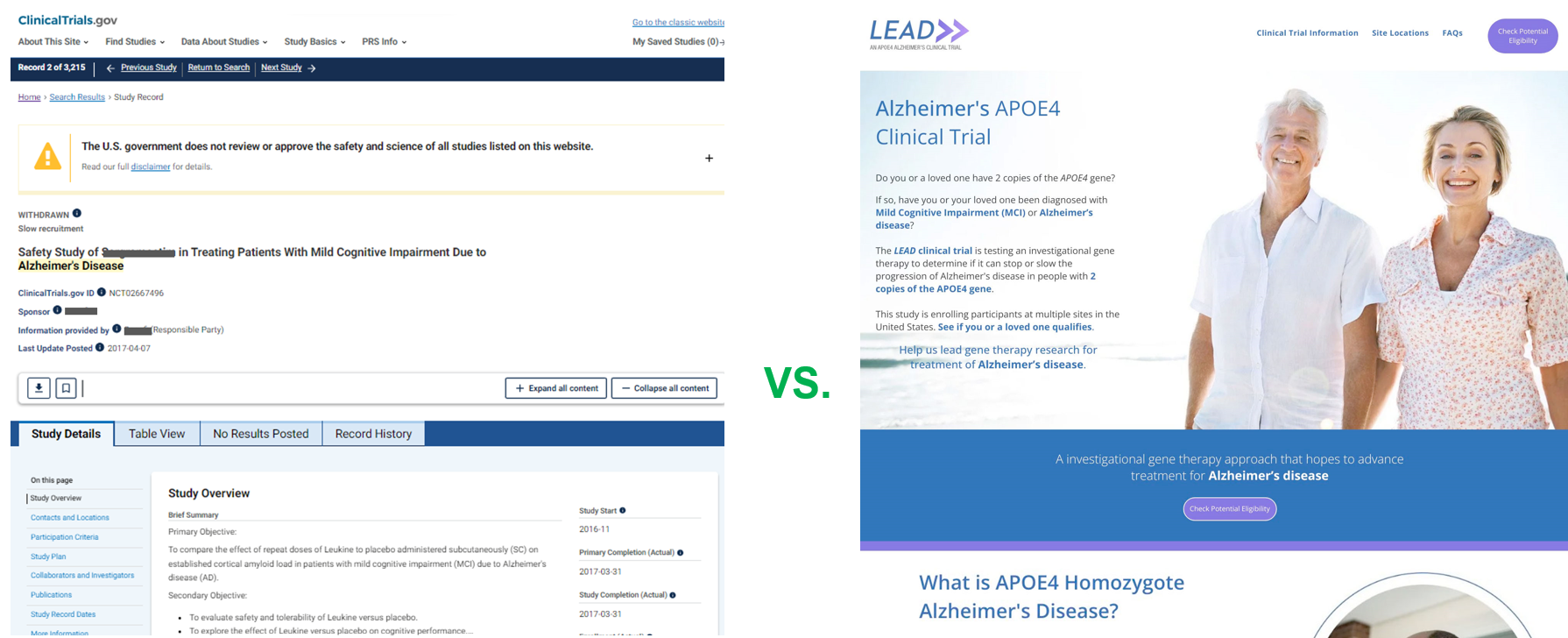
Sponsors have a choice. They can either let their study linger and get lost in ClinicalTrials.gov in hopes that someone stumbles upon it or create a dedicated clinical trial website design. This "point of convergence" can then serve the patient, their inner circle, HCPs, and physicians to embrace a trial in a more human approach. We know from our research that it only takes one friend or family member to scare a patient away from enrolling in the trial. "Oh, Mom, you shouldn't mess with a clinical trial. Who knows what they'll do to you." This regular occurrence may come from a place of good intent but is based on a lack of knowledge about the study. By creating a custom clinical trial website, it can be used as a resource and shared with everyone. The patient, their inner circle, and the physician all have a full understanding of the protocol and buy-in. By reimagining how clinical trials are presented, Sponsors can tout their research in new and exciting ways to patients while still adhering to IRB and HIPAA compliance.
Now imagine seeing your clinical trial on Google page # 1 in two spots. A sponsored ad, and a natural listing in the middle of the page result. When someone clicks, they are taken to the dedicated clinical trial website. A Facebook advocacy group awareness campaign can also still be employed in concert with Google. The difference is now you are leveraging a custom clinical trial website for ALL your marketing activities. Your new website asset can be used for all channels as the primary source of information and sharing.
ClinicalTrials.gov + Facebook is Not a Clinical Trial Marketing Strategy
Call it advertising, call it promotion, call it building awareness, credibility and trust but in the end, patients and physicians are human. We all need to be sold on the value of embarking on a medical procedure that may affect the outcome of our lives. A formal Google ads campaign and Google page # 1 search results (SEO) campaign to rank for the keywords that matter to your trial, plus a dedicated clinical trial website is the winning combination you need to increase awareness, sharing and yes, patient enrollment.
Pop-Quiz Questions for Sponsors, Investigators and CROs
1) After a patient is diagnosed, they are more likely to rush home and log onto Google rather than Facebook to seek answers, treatments, and alternatives – True or False?
2) A dedicated clinical trial website that explains the protocol in plain language, highlights the eligibility criteria, offers an FAQ section and shows where the sites are on a map would serve the patient, their inner circle, HCPs and even physicians more effectively than the scientific language listing on ClinicalTrials.gov? – True or False?
3) With regard to patient diversity, inner-city low-income people and/or people with poor reading skills are more likely to understand plain language laid out for them in a simplified clinical trial website than finding and deciphering a study listing on clinicaltrials.gov – True or False?
If you answered True to any of these questions, you are beginning to see missing puzzle pieces facing most studies.
Trial AMPlify, the leading patient recruitment agency for clinical trial marketing, is dedicated to one objective – Driving Patient Enrollment for Clinical Trials Worldwide. Contact us to see our patient recruitment campaigns in action and see why both major biopharma and start-up life science companies rely on us to help them surge patient enrollment. You'll be in good company.